Patients’ and caregivers’ “lived experience” crucial in CHS submissions on product access

It’s an exciting time in care for people with bleeding disorders. In recent years, a number of novel therapies have come through clinical trials and are entering the marketplace. Others are still in the pipeline. Before these products are reimbursed by our health system, however, they are subjected to health technology assessments (HTAs). In the case of coagulation products, the Canadian Hemophilia Society (CHS) makes submissions to the HTA bodies—Canadian Agency for Drugs and Technologies in Health (CADTH) and the Institut national d’excellence en santé et en services sociaux (INESSS) whose roles are to make recommendations to governments on access to novel therapies—to give the patient perspective on how the therapies may make a difference.
To prepare for this, in recent months the CHS conducted three separate “lived experience” surveys with people living with hemophilia A, hemophilia B and von Willebrand disease, and their caregivers. The surveys were conducted anonymously using the SurveyMonkey online platform. The existence of the surveys was made known via CHS social media networks, and CHS chapters were asked to encourage their members to participate.
Questionnaires were completed by 83 people, including:
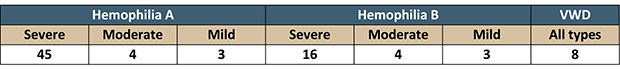
In order to provide CADTH and INESSS with the information they require for their assessments, the CHS used the same questions used in their consultations with all patient associations when evaluating novel therapies. Respondents took great care to answer in detail, and the CHS would like to thank them on behalf of the entire community.
Burden of disease
The first question asked people to describe how hemophilia A, hemophilia B or VWD impacts their (and/or their caregivers’) day-to-day life and quality of life. What aspects of the illness are more difficult than others? The 83 respondents provided 210 different answers which could be re-grouped into 47 similar issues, touching on health challenges, psychosocial issues, limits on normal activities and independence, work and school concerns, relationships, and economic strains. The 15 most common responses were (number of similar responses in parentheses):
- Limits to normal activities (22)
- Difficulty accessing veins (20)
- Damage to joints (16)
- Pain (14)
- Stress, anxiety, depression (13)
- Frequent bleeds despite prophylaxis (13)
- Worry about breakthrough bleeds (12)
- Time needed for infusions (11)
- Reduced mobility (9)
- Difficulty to exercise (8)
- Adhering to treatment schedule (7)
- High frequency of treatments (5)
- Cost of supplies, hospital visits (5)
- Time missed from work, school (5)
- Difficulty in travelling with factor supplies (4)
- Time to travel to hospital (3)
Current treatments
The second set of questions asked people to specify their current treatment and describe how they (or the people caring for them) are managing their bleeding disorder with currently available treatments. They were asked to consider benefits and side effects, and note any difficulties accessing treatment.
This is the portrait of the respondents’ treatment protocols:
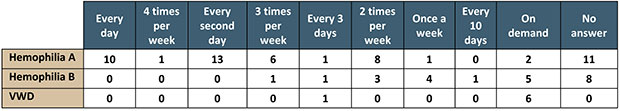
The respondents focused on the negative aspects related to their treatment, 24 of them in all. In order of frequency, they were:
- Difficulty accessing veins (30)
- Time lost from school/work for patient/caregiver (22)
- Long distance to clinic for treatment or to pick up factor supplies (11)
- Need for a Port-a-Cath or PICC line (7)
- Breakthrough joint bleeding (6)
- Short half-life of factor (6)
- Damaged veins (5)
- Frequent hospital visits (3)
- Side effects of treatment (allergic reactions) (2)
- Unable to follow prophylaxis schedule (2)
Satisfaction with treatment
We asked patients and caregivers how satisfied they are with the efficacy of their treatment. This is what they responded.
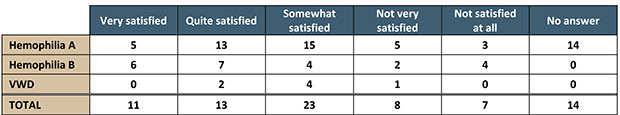
Of the nine different comments related to satisfaction, the most common were:
- Good efficacy to stop/prevent bleeding (13)
- Doesn’t last long enough (short half-life), high frequency of treatment (13)
- Breakthrough bleeds despite prophylaxis, insufficient protection (factor level) (7)
- Venous access difficulty (via port or vein) (5)
- Difficult/time consuming to administer (3)
Desired improvements
The last set of questions related to the improvements people would you like to see in a new treatment and how it might change quality of life. Respondents suggested 14 different kinds of improvements, of which the most desired were:
- Longer lasting treatment (half-life), less frequent administration (34)
- Better protection from bleeding (higher trough level) (20)
- No need for venous access (20)
- Easier mode of delivery (10)
- A cure (e.g. gene therapy) (5)
- Once-a-week treatment (5)
- Sub-cutaneous treatment (5)
- A pill (4)
- Constant factor level (3)
- Once-a-month treatment (2)
Of the 22 changes in quality of life these therapies might lead to, these were the 10 most often cited:
- Less worry, stress (8)
- Improved family quality of life, ability to travel (7)
- Less dependence on health services (6)
- Capacity to be more active (5)
- Less time lost from work (4)
- Less pain (3)
- Less damage to veins (3)
- Fewer visits to clinic (3)
- Less time needed to treat (3)
- Capacity to contribute more to society (2)
Conclusion
In the last few months, the CHS has used this valuable patient-reported data in three separate submissions to CADTH and INESSS on novel products for hemophilia A, hemophilia B and von Willebrand disease. We will again be using it in submissions we will make later in 2020.
We encourage people with bleeding disorders and their caregivers to continue to participate in similar CHS data collection exercises when you have the opportunity and, for MyCBDR users, to regularly report bleed/infusion information to your treatment centres, and to complete the PROBE questionnaire at least yearly. The patient perspective is critical to these therapies being made available.




