Hemgenix 4-year results show steady durability

MONTREAL, March 12, 2025 – Results from the HOPE-B trial for the hemophilia B gene therapy, Hemgenix, show steady expression of factor IX (FIX) four years after administration.
Dr. Steven Pipe, from the Hemophilia and Coagulation Disorders Program at the University of Michigan, presented 48-month results at the conference of the European Association of Haemophilia and Allied Disorders (EAHAD) in Milan, Italy in February.
The mean (average) FIX level in the 54 trial subjects 48 months after treatment was 37.4%, virtually unchanged from the results at 36 months, showing very promising long-term durability for the therapy.
The table below shows the different hemophilia B severity levels and the FIX levels achieved in the 54 participants.
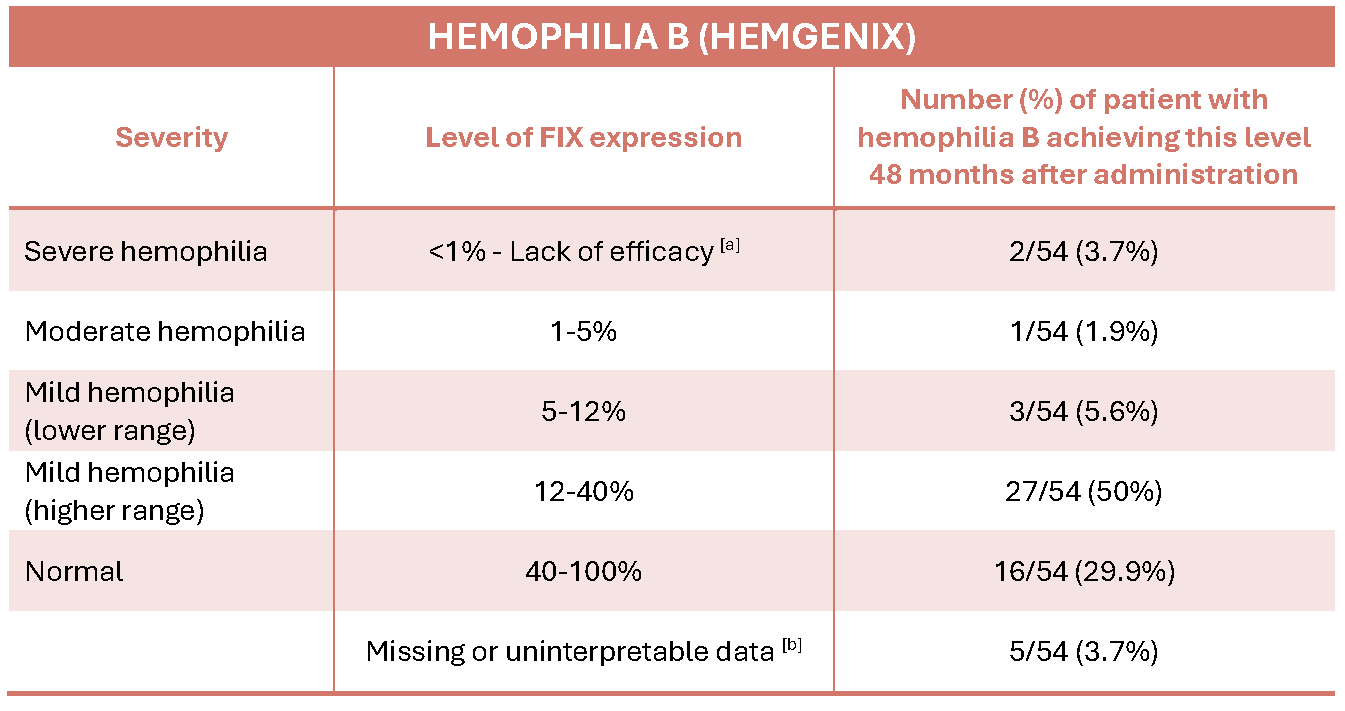
[a] Lack of efficacy of the gene therapy when there is no evidence of factor IX gene delivery and gene expression.
[b] The latest factor IX activity data are missing in 5 patients: one 77-year-old participant due to death (unrelated to etranacogene dezaparvovec) at 15 months after gene therapy; another following a liver transplant; a third after the patient returned to FIX prophylaxis; and two others whose last available factor IX assays were at 42 months.
Two subjects had no FIX expression after treatment, one returned to continuous FIX prophylaxis during Year 3 (all previously reported), and no subject resumed prophylaxis during Year 4.
Sixty percent (60%, 31/54) of the participants experienced no joint bleeds over the four years post-treatment; 46.3% (25/54) received no FIX infusions during that time.
No events of AAV5-associated genotoxicity have been confirmed, and no persistent liver toxicity events were observed. AAV5 is the viral vector used to deliver the FIX gene to the liver.
Hemgenix was approved by Health Canada in October 2023. Canada’s Drug Agency (previously CADTH) gave a positive recommendation regarding reimbursement in June 2024, conditional on price negotiations. Quebec’s INESSS gave a negative recommendation, pending longer-term data. The manufacturer, CSL Behring Canada, and the pan-Canadian Pharmaceutical Alliance (pCPA), on behalf of Canada’s public drug plans, began price negotiations in August 2024. These negotiations typically require six months.
For more information, see the CHS Gene Therapy Education Program at www.hemophilia.ca/gene-therapy.




